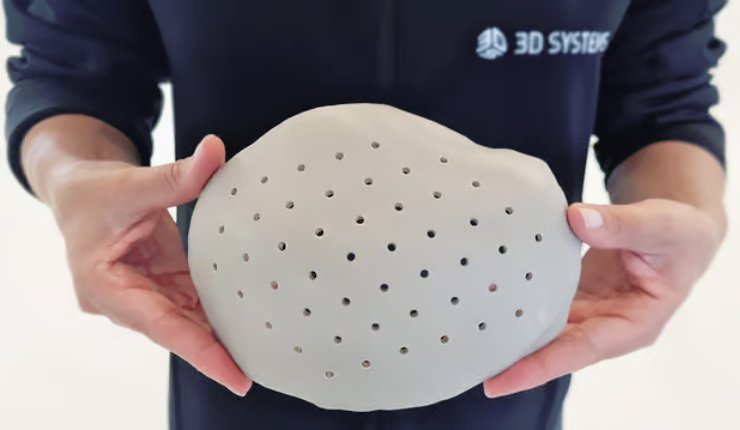
VSP PEEK Cranial Implant includes a complete FDA-cleared workflow comprising segmentation and 3D modelling software, the 3D Systems EST 220 MED 3D printer, Evonik VESTAKEEP i4 3DF PEEK (polyetheretherketone), and a pre-defined production process.
By utilising additive manufacturing solutions, this technology can produce patient-specific cranial implants with up to 85% less material than similar implants produced by traditional machining, according to 3D Systems, which can lead to ‘significant’ cost savings for an expensive raw material such as implantable PEEK.
The company says that the cleanroom-based architecture of the printer combined with simplified post-processing workflows makes it an ideal technology for producing patient-specific medical devices at the hospital site with faster turnaround while keeping the overall cost under control.
To date, this solution has been used to enable nearly 40 successful cranioplasties in Switzerland at University Hospital Basel, in Austria at Salzburg University Hospital, and in Israel at Tel-Aviv Sourasky Medical Center.