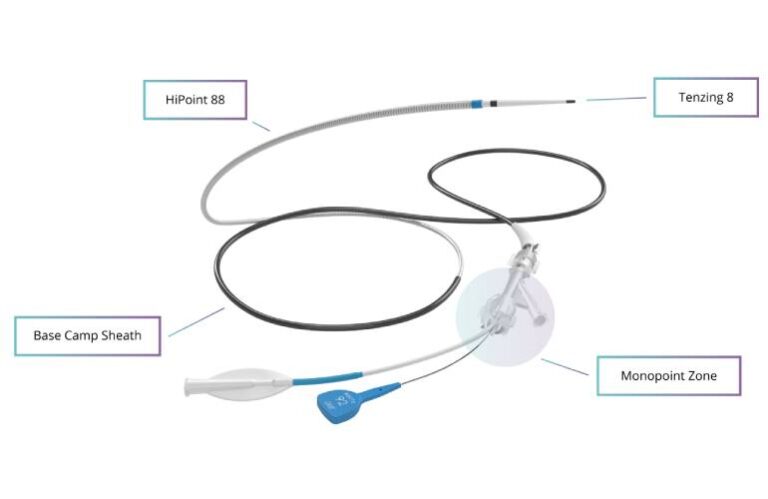
San Mateo, California-based Route 92’s HiPoint system includes the HiPoint 88 system powered by Tenzing 8, featuring the Monopoint approach. The company says it became the first neurovascular intervention company to win clearance for direct aspiration of a stroke-causing thrombus with a super-bore .088” catheter in the neurovasculature.
HiPoint 88 features an aspiration catheter (its 88 and 70 aspiration catheters), Tenzing 8 delivery catheter and Base Camp 2.0 sheath. It has a Monopoint approach, advancing from a single point of control. Tenzing-powered delivery enables atraumatic navigation through tortuous anatomy. It offers clinicians the flexibility to deliver vessel-matched, super-bore catheters to the M1. This also enables the rapid and efficient removal of large vessel occlusions — the cause of many acute ischemic strokes.