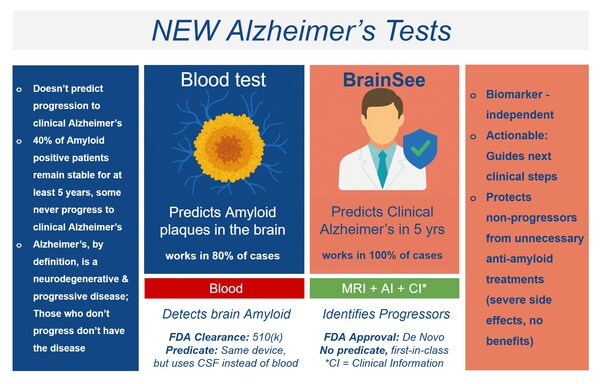
BrainSee is an AI-powered technology that combines standard brain MRI, basic cognitive assessments, age, and biological sex to generate a prognostic score. It is the first and only FDA-approved, non-invasive technology that offers clinicians actionable insight into whether patients over the age of 55 with amnestic mild cognitive impairment (aMCI) will progress to clinical Alzheimer’s disease within five years. The FDA granted BrainSee marketing authorization in January 2024 via the De Novo pathway.
Unlike traditional Alzheimer’s tests that detect non-specific biomarkers like amyloid beta plaques, BrainSee predicts disease progression. Roughly 40% of amyloid-positive aMCI patients remain stable for at least five years or return to normal cognition. These individuals are unlikely to benefit from anti-amyloid drugs and may face unnecessary side effects. BrainSee was developed to fill this critical gap by identifying those most likely to progress and guide them through the next steps aiming to prevent or delay the onset of dementia symptoms. Any licensed physician, not just neurologists, can prescribe the test. This includes primary care providers such as internists and family medicine doctors, as well as specialists in psychiatry, geriatrics, and preventive medicine. This broad accessibility is a major differentiator, enabling early cognitive risk assessment at the point of first contact—often in primary care settings—without the need for specialist referral.