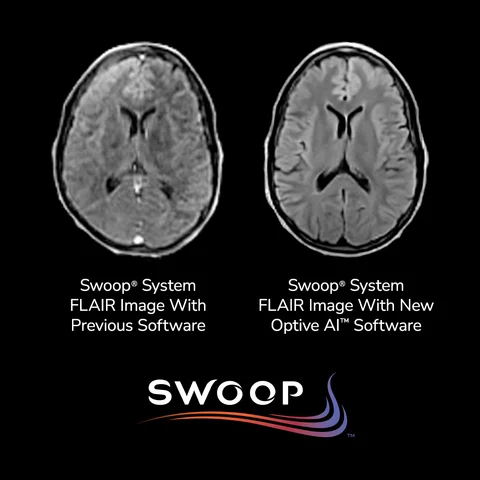
Hyperfine’s recent FDA clearance of its tenth-generation software, Optive AI™, marks a transformative leap forward in portable brain imaging technology. Integrated with the Swoop® Portable MR Imaging® system—the world’s first FDA-cleared AI-powered portable MRI—Optive AI™ uses advanced artificial intelligence algorithms to significantly enhance image quality in ultra-low-field magnetic resonance imaging. This innovation addresses a longstanding limitation in portable MRI by delivering clearer, more uniform, and anatomically detailed images, a breakthrough that brings ultra-low-field performance closer to that of conventional 1.5T scanners.
In clinical usage, Optive AI™ enhances every phase of the imaging process, from noise reduction and image acquisition to reconstruction and post-processing. This results in faster, more confident diagnostic decisions at the point of care, especially in emergency, bedside, and resource-limited settings. Early feedback from clinical sites has been overwhelmingly positive, with radiologists noting a marked improvement in image clarity and diagnostic confidence. These enhancements are critical for applications such as stroke detection, trauma assessment, and neurological monitoring, particularly when access to full-scale MRI units is delayed or impractical.