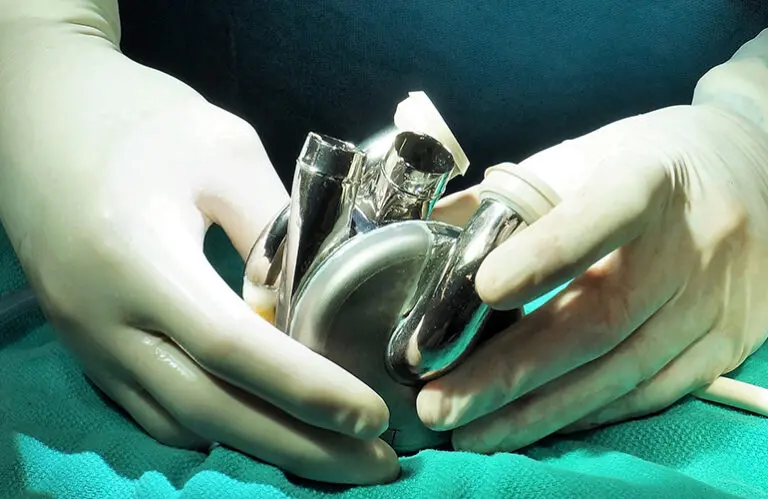
The designation supports the TAH as a bridge to transplant (BTT) for adults with severe biventricular or univentricular heart failure where current treatments, including LVADs, are not viable.
“This is more than a regulatory milestone. It’s a validation of a concept we’ve spent decades proving that a fully implantable, total artificial heart isn’t just possible, it’s necessary,” said Daniel Timms, founder and chief technology officer of BiVacor. “Patients with biventricular failure have been overlooked for too long. The early results from our clinical trial show that we can give them a second chance, without the compromises of older technologies.