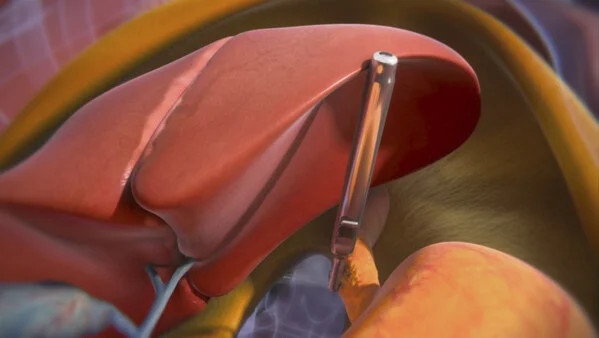
Levita Magnetics’ expanded FDA clearance for its Magnetic Surgical System (MSS) with MARS® represents a major advancement in minimally invasive surgery, especially for bariatric and hiatal hernia procedures. The integration of Dynamic Magnetic Positioning™ allows surgeons to internally retract the liver without additional incisions, using magnetic force to manipulate anatomy safely and effectively. The introduction of the new 12.5mm magnetic grasper further elevates this platform’s capabilities by offering enhanced access and control, particularly in patients with high BMI or challenging liver anatomy—areas where traditional tools often fall short.
This breakthrough addresses critical surgical needs by reducing incision count, improving visualization, and minimizing tissue trauma—leading to less postoperative pain, faster recovery, and fewer complications. The MARS system enhances surgical workflow while empowering clinicians to treat complex conditions more efficiently, whether in hospitals or ambulatory settings. As more surgeons adopt this magnetic-assisted approach, the potential to transform bariatric surgery outcomes and elevate standard care becomes increasingly clear, especially in a time when obesity-related surgical demand continues to rise globally.