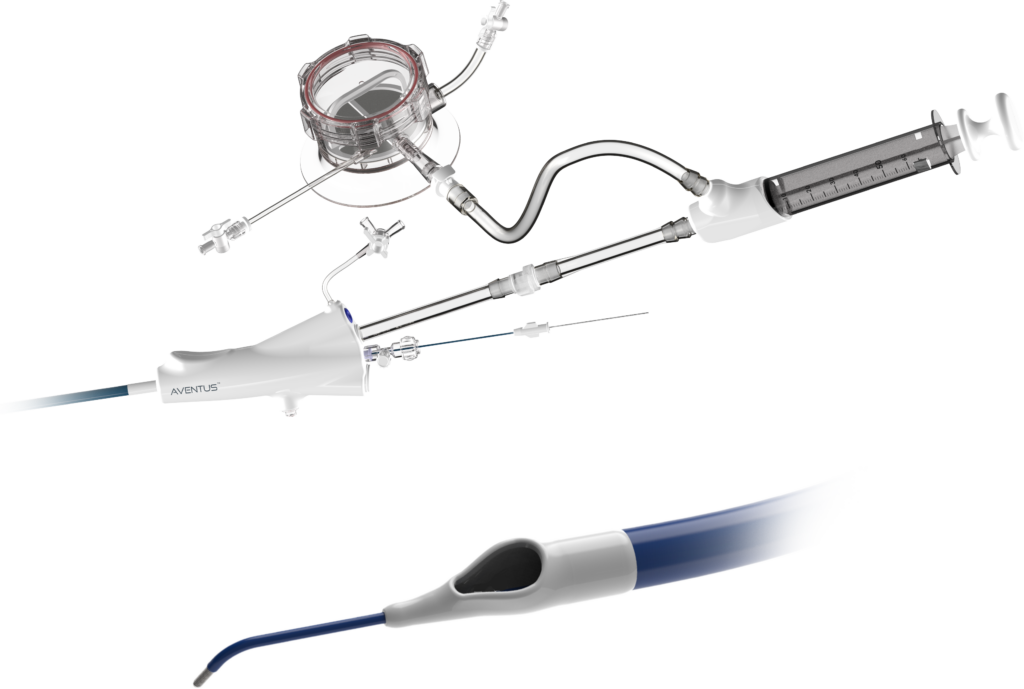
The expanded clearance means Aventus now has an indication to treat pulmonary embolism (PE). It comes around a month after the Menlo Park, California-based company shared positive study data for Aventus in PE. The study successfully met its primary endpoints, demonstrating safety and efficacy in treating acute intermediate-risk PE patients.
Aventus, a next-generation mechanical thrombectomy platform, features proprietary tissue-sensing technology. It provides operators with real-time information on the tissue composition in contact with the device. The system offers enhanced precision and control during procedures.