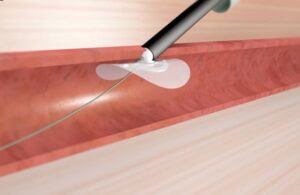
Galway, Ireland-based Vivasure designed the latest-generation PerQseal Elite for percutaneous vessel closure. The system leverages the legacy platform’s safety profile and ease of use while allowing for faster delivery. It aims to improve performance in calcium and the treatment of large-hole venous procedures, according to the company. That includes transcatheter mitral and aortic valve repair and replacement (TMVR/TAVR).
Vivasure labeled PerQseal the first sutureless, fully absorbable synthetic implant for large-bore vessel punctures. Placement occurs from inside the vessel. It makes deployment simpler and more controlled than conventional closure techniques. Additionally, the company says there are no fully bioresorbable devices available for closure following large-bore procedures.