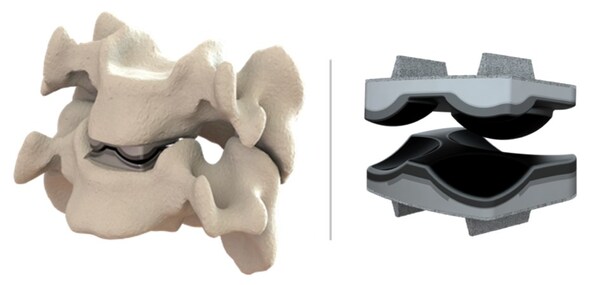
The IDE approval enables Dymicron to initiate a multi-center, prospective, historically controlled clinical trial comparing the safety and effectiveness of Triadyme-C to anterior cervical discectomy and fusion (ACDF) surgery in the treatment of symptomatic cervical disc disease (SCDD). The trial will enroll patients across several leading U.S. spine centers, with the first implantations expected in Q4 2025.
“This FDA approval is a value-defining achievement for Dymicron,” said Alan S. Layton, Chief Executive Officer and Chairman of the Board at Dymicron. “It reflects both the strength of our technology and the disciplined execution of our regulatory roadmap. We are now poised to generate high-quality clinical data that will support a future PMA submission and lay the groundwork for commercialization in the U.S. market.”