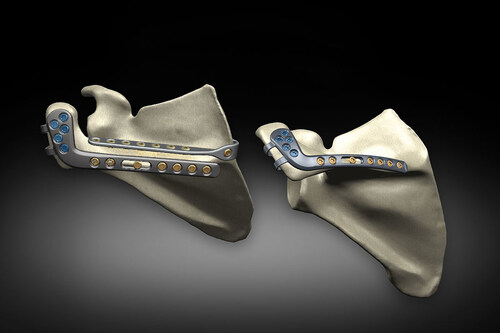
Acromial and scapular fractures are a rare but devastating complication that can occur among every rTSA implant design. The reported rate of acromial and scapular fractures after rTSA averages 2.8 percent but can be as high as 10.9 percent for some patient diagnoses.2 There is currently no accepted treatment solution for patients with this challenging rTSA complication. Recognizing this unmet clinical need, Exactech developed a novel solution to help surgeons treat this unique complication.
Designed by Jonathan Levy, MD, Howard Routman, DO, Peter Cole, MD, George Athwal, MD, Michael McKee, MD, and Joaquin Sanchez-Sotelo, MD, PhD, the Equinoxe Scapula Reconstruction System is the only trauma solution designed to treat acromial and scapular fractures with rTSA in mind.