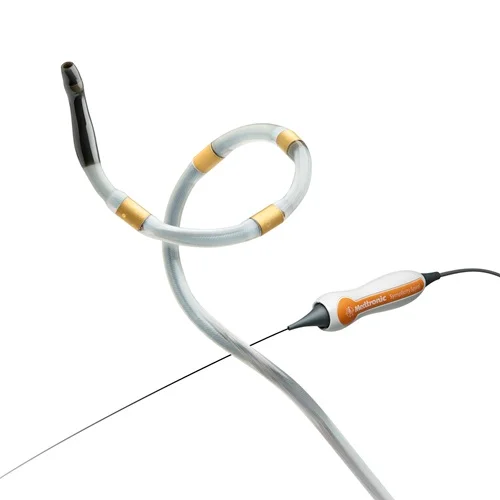
Medtronic has officially commenced its groundbreaking SPYRAL GEMINI Pilot study, marking a pivotal step in the evolution of hypertension treatment through multi-organ denervation (MDN). The study, which recently treated its first patient in Tupelo, Mississippi, investigates the novel concept of combining renal and hepatic artery denervation using the Symplicity Spyral™ catheter. Building on the clinical success of renal denervation, this next-generation approach targets additional sympathetic nerve-rich vascular regions, such as the common hepatic artery, to enhance therapeutic impact in patients with uncontrolled hypertension. The Symplicity Spyral system, known for its low-profile, single-catheter design, is central to this innovation, offering a streamlined method for delivering MDN with high precision.
The SPYRAL GEMINI Pilot is a multicenter, international feasibility trial enrolling up to 175 patients across the U.S., Europe, and Australia. It includes two parallel cohorts—patients off antihypertensive medications (“OFF MED”) and those on such medications with high cardiovascular risk (“ON MED”). The study will assess both safety and early efficacy of this dual-targeted denervation strategy, with evaluations beginning at three months and continuing up to 36 months post-procedure. This initiative follows promising preclinical data presented at EuroPCR 2025, which demonstrated a 90% reduction in norepinephrine—an indicator of sympathetic nervous activity—validating the potential of hepatic artery denervation when combined with renal denervation.