MDR certification is the EU’s rigorous regulatory process for ensuring the safety and performance of medical devices, including software like SwiftMR that interacts with imaging systems. This comprehensive evaluation conforms with applicable regulatory requirements.
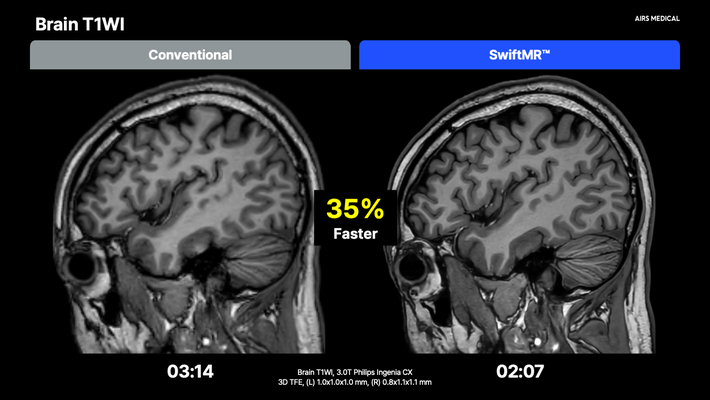
SwiftMR’s MDR certification now extends to all body parts — including abdomen, breast, prostate, and pediatric imaging — in addition to previously approved applications for brain, spine, and musculoskeletal imaging. This designation further expands SwiftMR’s clinical utility across a wide range of MRI exams.
“Earning expanded MDR certification reflects AIRS Medical’s commitment to delivering solutions that hold up to the world’s most demanding regulatory standards,” said Jayden Jung, Head of EMEA. “Today, faster MRI is more accessible across Europe than ever before.”