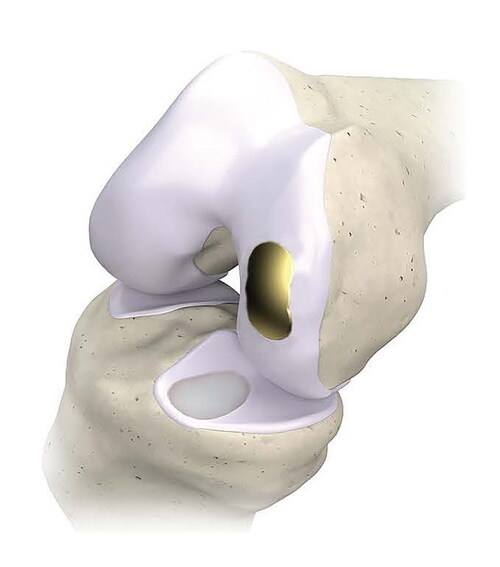
“My career has been defined by osteochondral allografts. At some point, biology fails and no longer becomes a good option for a patient, but it’s far too early for any kind of joint replacement,” said Riley J. Williams III, MD, Chief of the Sports Medicine Institute at the Hospital for Special Surgery in New York City. “Overture is bridging that gap between biologic options and joint replacement with an implant that resurfaces the joint, providing a durable, predictable, and lasting solution that adheres to the principles of joint preservation.”
The OvertureTi Knee Resurfacing System received 510(k) clearance from the United States Food and Drug Administration in March 2023. To date, approximately 150 cases have been successfully completed in a limited market release that commenced in November 2023.