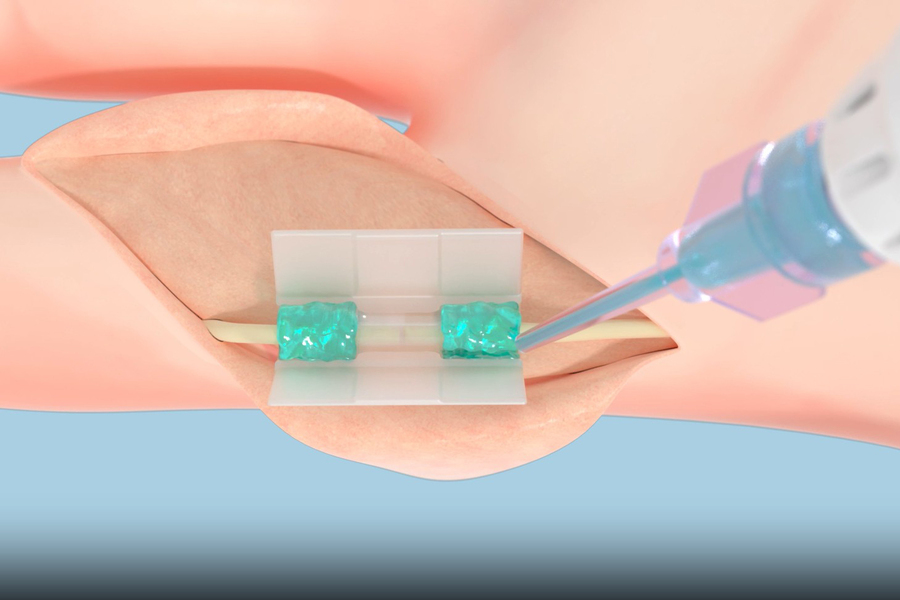
When surgeons repair tissues, they’re currently limited to mechanical solutions like sutures and staples, which can cause their own damage, or meshes and glues that may not adequately bond with tissues and can be rejected by the body.
Now, Tissium is offering surgeons a new solution based on a biopolymer technology first developed at MIT. The company’s flexible, biocompatible polymers conform to surrounding tissues, attaching to them in order to repair torn tissue after being activated using blue light.
“Our goal is to make this technology the new standard in fixation,” says Tissium co-founder Maria Pereira, who began working with polymers as a PhD student through the MIT Portugal Program. “Surgeons have been using sutures, staples, or tacks for decades or centuries, and they’re quite penetrating. We’re trying to help surgeons repair tissues in a less traumatic way.”
In June, Tissium reached a major milestone when it received marketing authorization from the Food and Drug Administration for its non-traumatic, sutureless solution to repair peripheral nerves. The FDA’s De Novo marketing authorization acknowledges the novelty of the company’s platform and enables commercialization of the MIT spinout’s first product. It came after studies showing the platform helped patients regain full flexion and extension of their injured fingers or toes without pain.