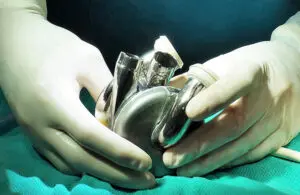
TAP provides early and frequent strategic engagement from the FDA, patients, providers and payers. It facilitates rapid development and widespread access to medical devices. The company expects its acceptance into the program to help accelerate its novel titanium TAH system through engagement with the agency through the entire product life cycle. Acceptance comes a few months after the FDA granted breakthrough device designation for the system.
Huntington Beach, California–based BiVacor designed the TAH to replace the function of the native heart completely. Sized similarly to an adult fist, it uses magnetic levitation technology. Left and right vanes positioned on a common rotor form the only moving part, a magnetically suspended double-sided centrifugal impeller.