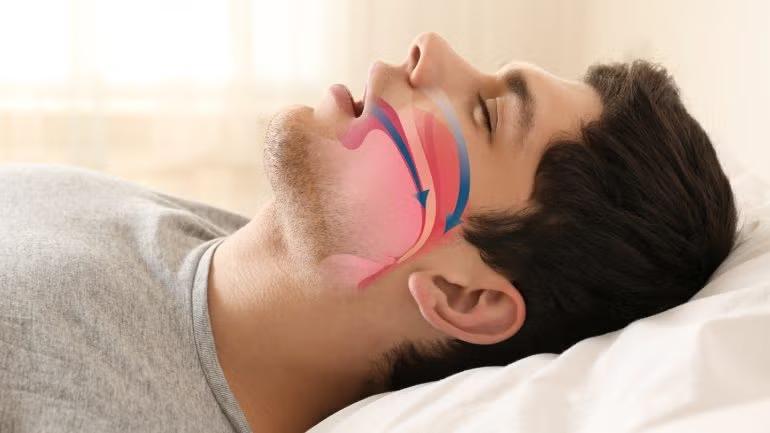
The system is indicated for use in those having an Apnoea-Hypopnea Index (AHI) between 15 and 65.
Genio’s approach to hypoglossal nerve stimulation (HGNS) sets it apart with its bilateral stimulation capability.
It is a leadless system that is compatible with 1.5T and 3T magnetic resonance imaging (MRI) scans and eliminates the need for an implanted battery.
The Genio system is powered by an upgradable wearable component, allowing patients to benefit from future technological improvements without the need for additional surgical interventions.