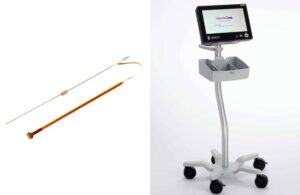
The OneRF system creates radiofrequency (RF) lesions for the treatment of pain or for lesioning nerve tissue for functional neurosurgical procedures. Eden Prairie, Minnesota–based NeuroOne initially submitted the system ahead of schedule in April. It now targets a limited commercial launch in the fourth quarter of calendar year 2025.
Trigeminal nerve ablation, a minimally invasive procedure, uses RF energy to destroy abnormal tissue and relieve severe, chronic pain in the face caused by trigeminal neuralgia. Using its patented OneRF platform, NeuroOne designed its ablation procedure to provide pain relief for this condition.