In a study published in Nature, a research team led by Prof. Han Shuo from the Center for Excellence in Molecular Cell Science (Shanghai Institute of Biochemistry and Cell Biology) of the Chinese Academy of Sciences applied proximity labeling to immunomodulation for the first time and developed a novel cell-surface protein engineering strategy named Proximity Amplification and Tagging of Cytotoxic Haptens (PATCH), solving the key bottlenecks in immunotherapy.
Proximity labeling, a technique typically used for detecting the spatial relationships of proteins, is an innovative approach from the perspective of chemical biology. Researchers reimagined proximity labeling as a functional modulation tool. Their goal was to directly amplify targeting signals on the tumor cell surface, thereby marking the cells that need to be attacked by the immune system.
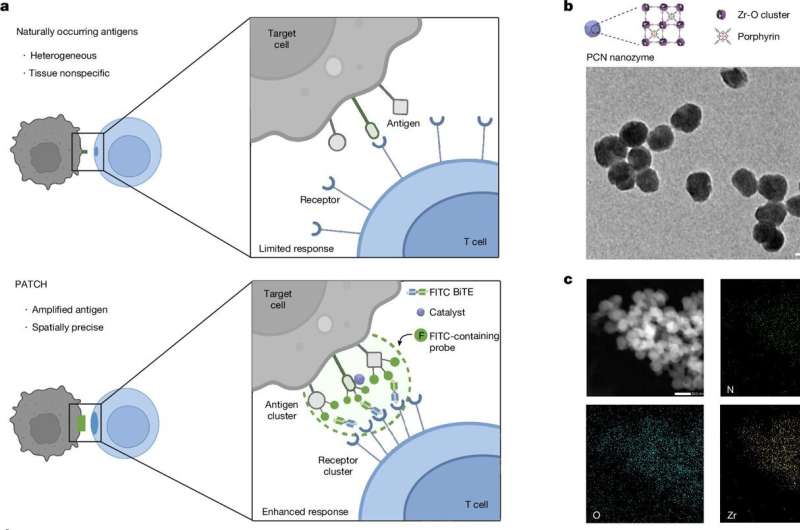
In the newly developed PATCH strategy, an engineered nanozyme (PCN) was first delivered to the surface of tumor cells, and th