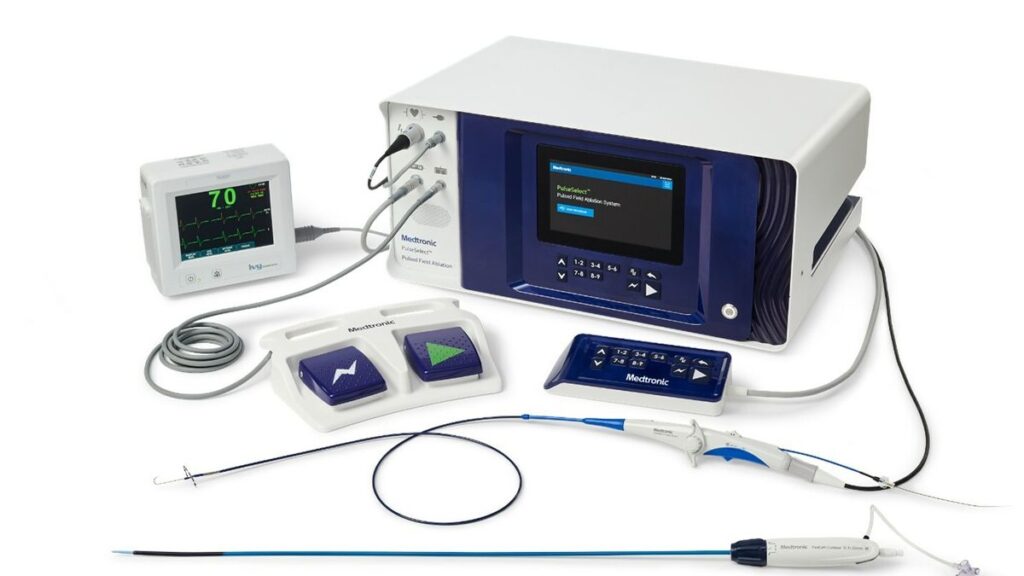
The minimally invasive, cardiac ablation system is indicated for the treatment of paroxysmal and persistent atrial fibrillation (AFIb).
Medtronic said it will start commercialization in early 2024.
“Launching the first FDA-approved PFA technology is not just a milestone; the PulseSelect PFA system is setting a new standard in safety for AF ablation with excellent efficacy and efficiency,” Medtronic SVP and Cardiac Ablation Solutions President Rebecca Seidel said in a news release. “It’s a major step towards fulfilling our vision of providing disruptive electrophysiology solutions for patients.”
“The PulseSelect PFA system, together with the CE Marked Affera mapping and ablation system and our strong Cryo platform, enables us to provide a broad portfolio of solutions to clinicians and their patients, all developed with years of research and supported by compelling scientific evidence,” she continued.