TORONTO–(BUSINESS WIRE)– Xpan Inc., a medical device company focused on minimally invasive surgical innovation, is proud to announce 510(k) clearance from the U.S. Food & Drug Administration for the XpanTM Universal Trocar System, and successful completion of initial clinical procedures in the United States.
The FDA clearance and initial cases are significant milestones in the company’s journey to transform the standard of care for minimally invasive surgical access. Xpan is excited to bring its innovative technology to the U.S. market and looks forward to improving the quality of care for patients.
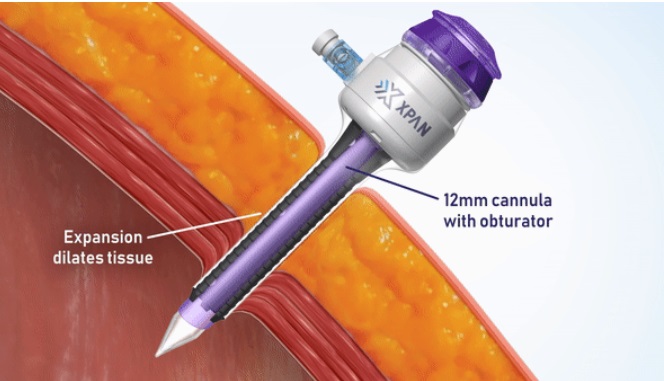
The XpanTM Universal Trocar System is a unique, patented radially expandable access device that initially enters the body as a 3 mm port which can subsequently be dilated intra-procedurally to 5 mm and/or 12 mm at the surgeon’s discretion as required by the demands of the procedure. The product allows surgeons to utilize the most minimally invasive instruments and techniques while minimizing patient tissue disruption and morbidity via radial dilation versus cutting of the tissue when surgeons require larger instruments in critical situations. It is estimated there are more than 5 million abdominal and thoracic minimally invasive procedures in the United States alone that can benefit from the Xpan’s Universal Trocar System.