The beginning of the study follows FDA investigational device (IDE) approval. Tandem II, a prospective, multi-center, non-randomized, single-arm study looks at the safety and performance of Duo in patients with severe or greater symptomatic tricuspid regurgitation (TR).
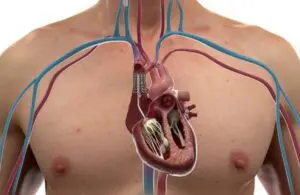
Duo features a coaptation valve that works in tandem with the native tricuspid valve to restore valve function. Delivered using percutaneous techniques, Duo is secured using a novel anchor system. This anchor system leaves the right heart and native valve apparatus untouched.