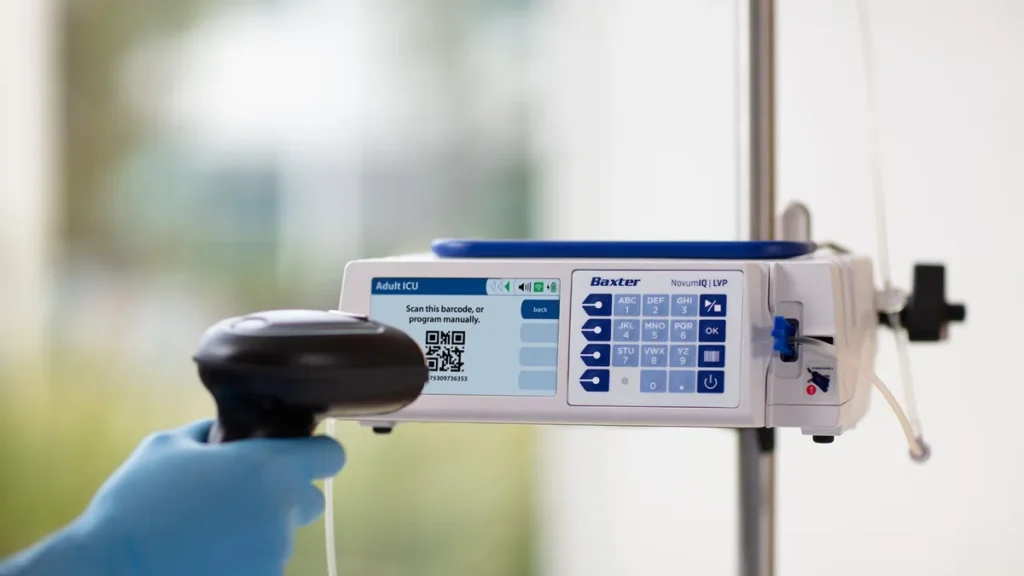
Baxter has received 510(k) clearance for its large volume infusion pump (LVP) Novum IQ, the company said Monday.
The clearance follows three years of talks with the Food and Drug Administration, in which time a delay to the authorization drove Baxter to wipe $100 million in sales off its forecast in 2022. A Baxter spokesperson said in an email that the company will update 2024 guidance on its upcoming first-quarter earnings call and expects Novum IQ to be a meaningful contributor to future growth.
Evercore ISI analysts said the success of Baxter’s Spectrum pumps and the return of BD’s Alaris to the market means the $100 million annual sales assumption no longer applies. Even so, Evercore and Stifel analysts called the clearance positive for Baxter in notes to investors