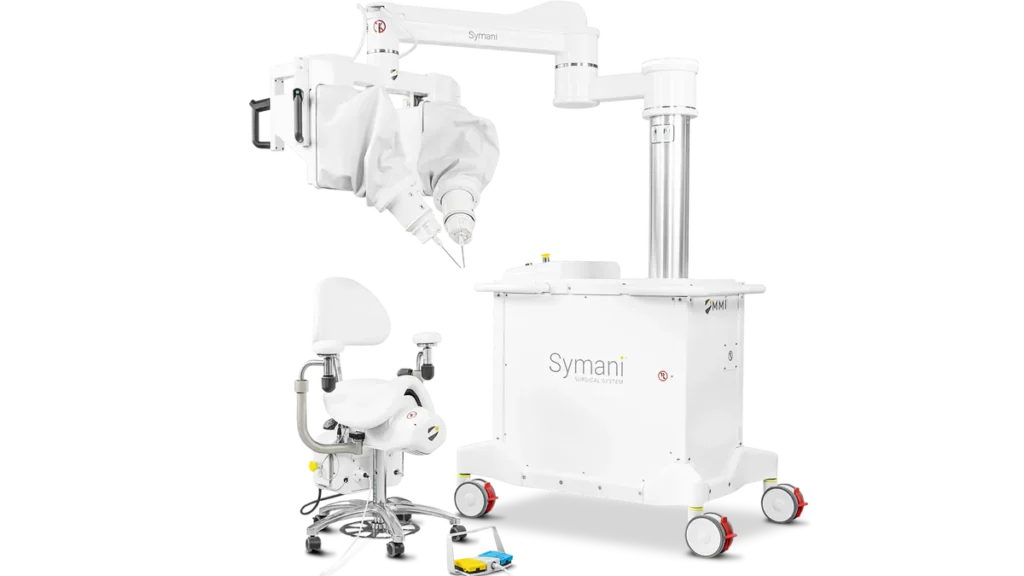
Medical Microinstruments (MMI) has received de novo authorization for a robotic system that enables surgeons to reconnect tiny blood vessels, the company said Monday.
The Food and Drug Administration authorized the device, called the Symani Surgical System, for soft tissue manipulation to perform microsurgery, a way to restore blood flow and redirect fluid during reconstruction or repair.
CEO Mark Toland said the commercial availability of Symani could increase the number of physicians who can perform complicated microsurgical procedures. MMI plans to immediately launch the system in the U.S.