Receiving the UKCA and CE Mark approval underscores Meditrina’s dedication to meeting the highest standards of quality, safety, and efficacy required for market entry in the European Union and the UK. This achievement not only validates the company’s commitment to regulatory compliance but also positions Meditrina as a trusted provider of cutting-edge medical solutions.
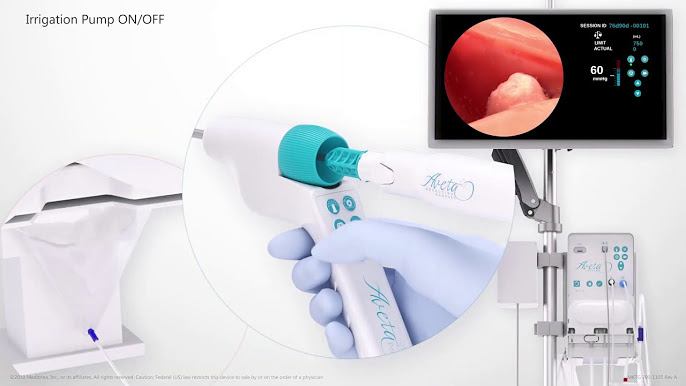
Hysteroscopy is a crucial diagnostic and therapeutic procedure used to evaluate and treat conditions affecting the uterus. Meditrina’s Aveta hysteroscopy system combines advanced technology with ergonomic design, enabling healthcare professionals to perform minimally invasive procedures with enhanced visualization and control, ultimately leading to improved patient outcomes and experiences.
“We are excited to receive UKCA and CE Mark approval for our hysteroscopy system, as it validates the exceptional quality and performance of our product,” remarked Csaba Truckai, CEO of Meditrina. “Furthermore, the successful completion of our initial international procedures in Spain and Denmark signifies a significant milestone in our mission to revolutionize women’s healthcare on a global scale. We are proud to collaborate with healthcare providers worldwide in delivering advanced solutions that empower women to lead healthier lives.”