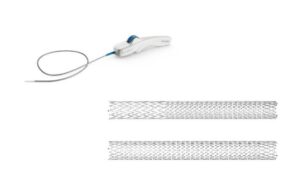
Duo, an implantable medical device, treats symptomatic venous outflow obstruction in patients with chronic venous insufficiency (CVI). Amsterdam-based Philips added Duo to its portfolio when it acquired Vesper Medical in 2021.
On June 11, Dr. Erin Murphy of the Sanger Heart & Vascular Institute, Atrium Health, in Charlotte, North Carolina, successfully used Duo for the first time outside a clinical trial. Murphy also served as an investigator in the VIVID study of the stent, which contributed to the device’s FDA approval.
Duo features two stents — the Duo Hybrid and Duo Extend — of various sizes, Philips said in a news release. It has a distinct, integrated design that combines multiple zones of differing mechanical properties into a single stent. For long lesions, Duo Extend smoothly overlaps with Duo Hybrid to extend therapy. The stents work together and minimize the risk of fracture and corrosion while catering to caudal veins with smaller diameters.