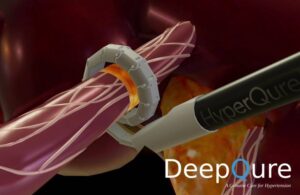
The Seoul, South Korea–based company designed HyperQure to perform renal denervation (RDN) through an extravascular (laparoscopic) approach. DeepQure says it’s the world’s first extravascular RDN medical device for treating resistant hypertension.
Though it faced clinical trial challenges to get there, RDN for hypertension became a hot space in medtech over the past decade, with several big names developing their own technology. Recor Medical became the first company to win FDA approval for RDN for hypertension back in November 2023. Medtronic received approval for its Symplicity Spyral system just weeks later.