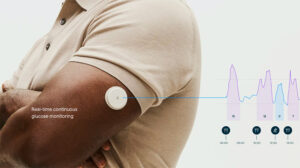
The company built both systems on its FreeStyle Libre CGM technology, but both aim to meet different needs. Lingo offers consumers a solution to better understand and improve their health and wellness. Libre Rio provides monitoring for adults with type 2 diabetes who aren’t on insulin and manage diabetes through lifestyle modifications.
Abbott acknowledged this month that Lingo’s clearance came on May 29, but the Libre Rio sensor marks another entry into the over-the-counter CGM market. Like the companies’ prescription CGMs, Libre Rio will rival Dexcom’s Stelo for people with type 2 diabetes who do not use insulin.