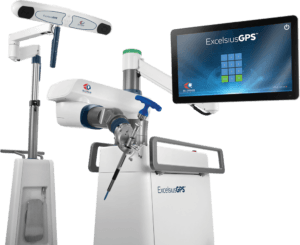
Audubon, Pennsylvania-based Globus Medical received the 510(k) clearance on June 14 for the robotic positioning system, which was designed to help surgeons position implant components and prepare bony anatomy during orthopedic procedures.
“We are ramping up production and targeting commercial release in the fourth quarter,” a Globus spokesperson told MassDevice today.
“We are excited to have received 510(k) clearance by the FDA for ExcelsiusFlex with Total Knee Arthroplasty (TKA) application,” the spokesperson said in a email. “This new robotic navigation platform joins the already best-in-class Excelsius Ecosystem, designed to offer surgeons enhanced control, resection accuracy, and procedural flexibility to Total Knee Arthroplasty.”