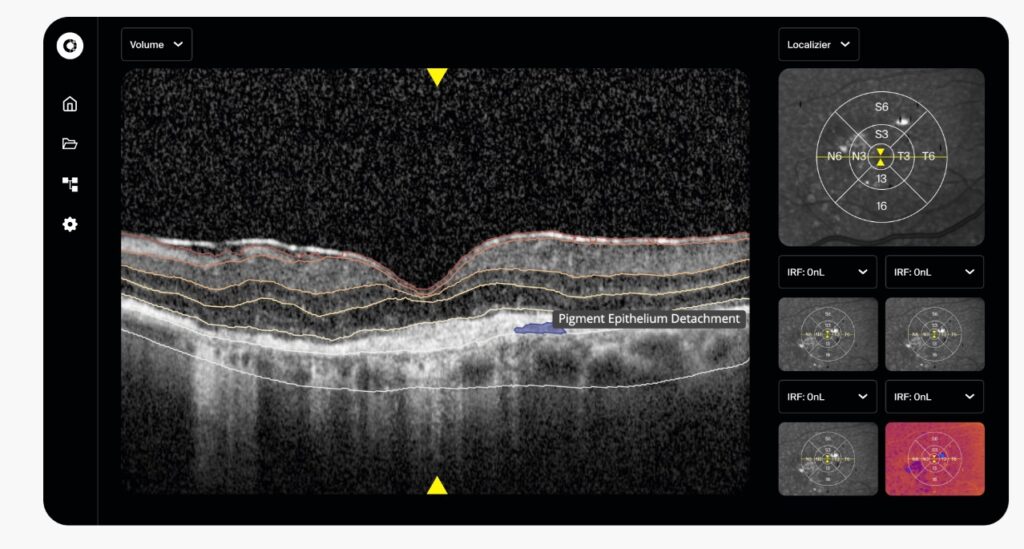
The Certificate applies to RetinAI Discovery®, a transformative healthcare platform to unlock the power of digital data in Ophthalmology, and the AI-based models to identify and quantify retinal layers, retinal fluids and retinal biomarkers to aid in the diagnosis and monitoring of diseases in Ophthalmology.
“The EU-MDR is among the world’s most robust regulatory frameworks for healthcare technology, with the highest standards on the clinical investigation and sale of medical devices for patients,” said Dr. Carlos Ciller, CEO and co-founder of Ikerian and RetinAI. “The significant number of AI-based certified products we have obtained in Ophthalmology highlights our dedication to innovation in the space to maintain high-quality standards in the development of our products, together with our compliance of GDPR data privacy and security laws in the EU. We are committed to providing top-notch healthcare data and AI solutions to patients and healthcare providers.”