CamDiab retained MCRA’s team of Digital Health and Quality Assurance experts to assist with the FDA submission process for the CamAPS FX app. Due to the nature of the software used, the MCRA team assembled a pre-determined change control plan (PCCP) to ensure the software could be updated easily, without the need for additional FDA submissions. The pre-determined change control plan is an impressive achievement for MCRA and CamDiab, as it is a relatively new concept from the FDA with a high-level of expertise needed.
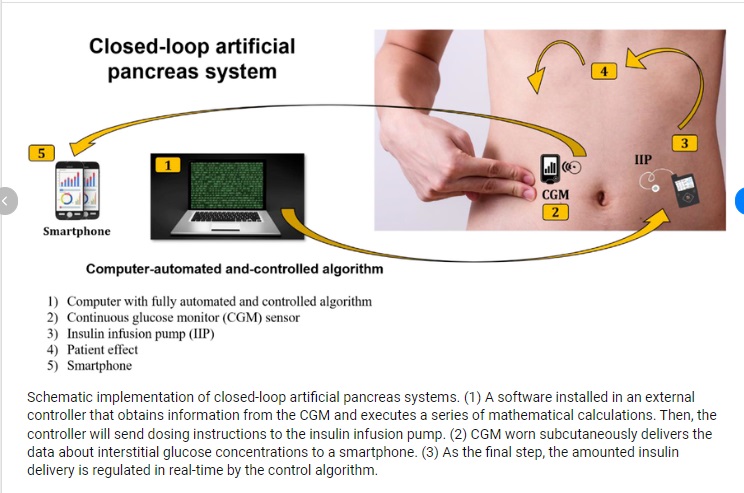
The CamAPS FX is an advanced adaptive hybrid closed-loop app that acts as an ‘interoperable automated glycaemic controller device’ (iAGC). The Android app – known as the world’s first artificial pancreas app – helps to manage glucose levels in people with type 1 diabetes, aged two and older, including during pregnancy. The app allows a compatible insulin pump and a compatible continuous glucose monitor to ‘talk to each other’, creating an artificial pancreas.