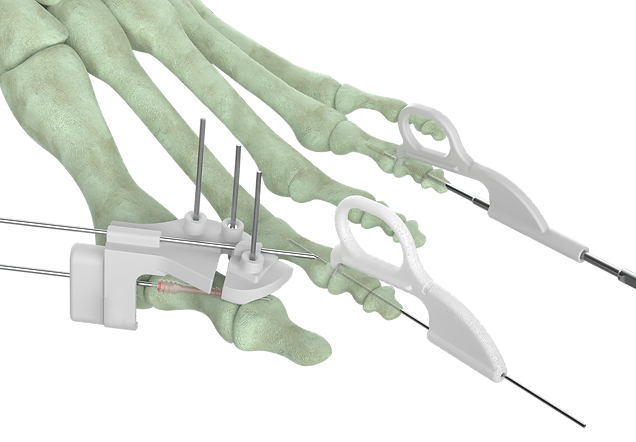
Forma Medical is focused on bunion-adjacent procedures, and with the OptimalMTP® Plating System approval is positioned to address the evolving needs of surgeons and patients. Forma Medical aims to set new standards for MIS solutions by providing tailored solutions, enhancing efficiency and efficacy in MIS foot and ankle surgery.
At the forefront of the Optimal Plating System launch is OptimalMTP®, the first plate and screw system dedicated to less invasive MTP Fusion of the first ray. This flagship system along with Forma’s current forefoot offering, OptimalHT® and OptimalAkin showcase Forma Medical’s unwavering commitment to innovation and patient-centric solutions, empowering surgeons to address complex orthopedic challenges with unparalleled reproducibility and efficiency in a far less invasive manner than the current standard of care.