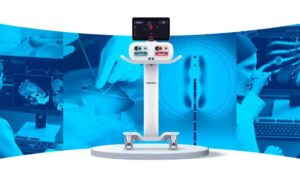
Varian designed IntelliBlate to provide greater predictability, precision and control during the ablation of soft tissue. The system produces large, controlled, spherical ablation zones for predictable treatment delivery. Users can deliver ablation with a single probe or dual probes, each linked with independent controls. The system’s Ximitry probe enables new features like laser disk alignment and intuitive LED indicators, too.
According to a news release, Varian’s ablation system design and intuitive interface deliver a portable, flexible system. Modular elements enable clinicians to expand their treatment capabilities to meet changing clinical demands, too. Despite changes and flexibility, users can still control operational efficiency throughout the ablation procedure