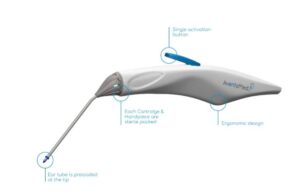
Solo+ combines multiple steps into an all-in-one device for a simplified approach to enable office-based pediatric tympanostomies. To date, ear tube placement typically takes place in an operating room and requires general anesthesia. The company designed the system to eliminate the need for general anesthesia, offering a quicker recovery and minimizing disruptions.
With the single-use system, ear, nose and throat (ENT) specialists can place the ear tube in the patient’s eardrum with just the press of a button. By pressing the button while using as little as topical anesthesia, ENT specialists can perform these placements in alternative sites of care. AventaMed says its offering helps address unmet needs while improving the patient experience.