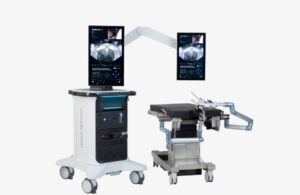
Hydros delivers the San Jose, California-based company’s Aquablation therapy. The company received FDA investigational device exemption (IDE) for its Aquablation procedure in September 2023. Aquablation uses waterjet resection to precisely eradicate prostate tissue. It provides the potential for an effective cancer treatment while maintaining the patient’s quality of life.
The image-guided, automated, heat-free robotic therapy uses real-time ultrasound imaging. This provides the surgeon with a multi-dimensional view of the prostate. Aquablation therapy enables personalized treatment planning tailored to each patient’s unique anatomy, Procept Biorobotics says.