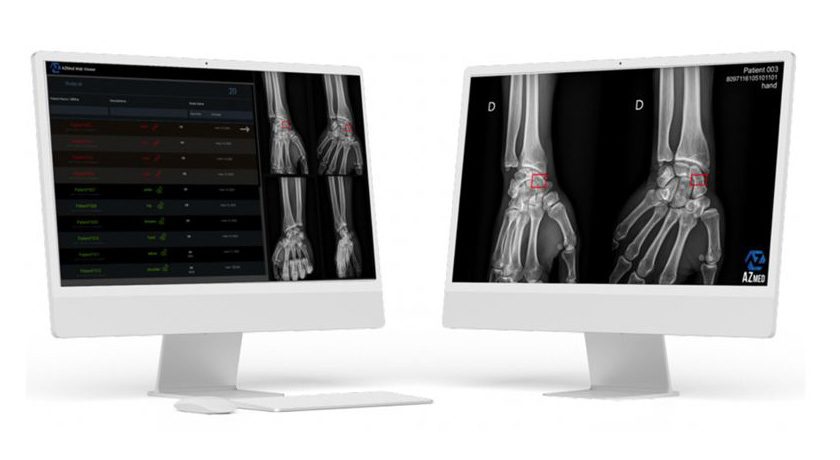
“The 510(k) clearance reflects our commitment to meeting the needs of healthcare professionals,” said Julien VIDAL, CEO of AZmed. “We are excited to extend our innovation to pediatric care, empowering clinicians with advanced tools to achieve the best outcomes for their patients.”
Through rigorous bench testing, the study with SimonMed Imaging confirmed Rayvolve’s efficacy in clinical settings, further validating AZmed’s commitment to collaborating with healthcare leaders to advance medical imaging technology. Involving a dataset of 3,000 pediatric radiographs, the study demonstrated Rayvolve’s high sensitivity (96%) and specificity (86%), making it one of the most effective software for assisting radiologists in detecting fractures in children, with an Area Under the Curve (AUC) of 94%.
“We are proud to have contributed to validating Rayvolve’s effectiveness,” said Dr. Sean Raj, Chief Innovation Officer of SimonMed Imaging. “This study underscores the transformative potential of AI as an indispensable diagnostic tool for pediatric fracture detection.”