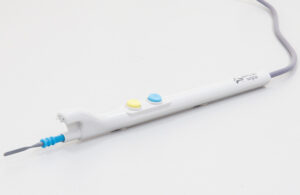
The latest clearance builds on the company’s Ultravision2 system, cleared in 2023 for the removal of smoke and particulate matter generated by electrosurgical equipment during laproscopic operations.
Cardiff, U.K.–based Alesi Surgical says its Ultravision2 system is now cleared for all open, laparoscopic, and robotic procedures that create surgical smoke.
“We have been overwhelmed with the positive surgeon feedback we have received during the development of the IonPencil,” Alesi CEO Dominic Griffiths said in a statement shared with MassDevice. “The hazards of surgical smoke in the operating room are increasingly recognized, and we are delighted to be able to offer U.S. surgeons and nurses this new, unique, and innovative way of addressing the problem.”