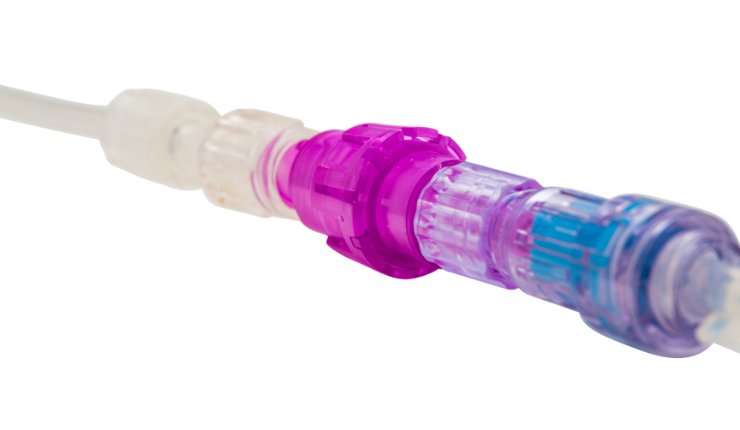
Linear Health Sciences, a medical device company that has developed a proprietary, breakaway safety valve platform, has announced that it received an expanded indication for its Orchid Safety Release Valve (SRV) from the U.S. Food & Drug Administration (FDA).
The Orchid SRV is a sterile, single-use connector for needle-free access that makes return to treatment fast, simple, and clean, and improves both the patient and clinician experience. It is indicated for patients two weeks of age and older during intermittent or continuous infusion, and can now be used during the transfusion of blood or blood products.
“The Orchid SRV has been shown to significantly reduce IV dislodgement and its associated costs and complications,” said Rob Headley, Vice President of Sales and business development at Linear Health Sciences. “This, our third expanded indication, reflects a significant shift in the potential for a new gold standard of care, creates a valuable functional moat competitively, and expands our total addressable market exponentially.”