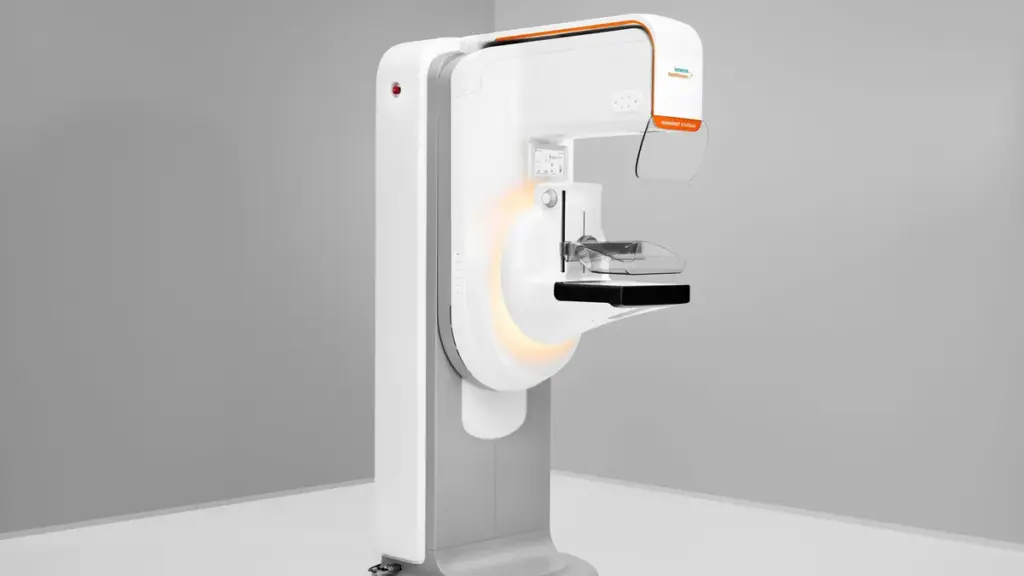
Siemens received 510(k) clearance for its Mammomat B.brilliant mammography system in April. The Food and Drug Administration clearance covered elements involving 2D breast imaging, breast biopsy and titanium contrast-enhanced mammography. Separately, Siemens filed for premarket approval of the 3D portion of the system.
The FDA has now approved the 3D elements, which feature a 50-degree wide-angle technology. Siemens said the angle is the widest available.
The mammography system has a scan time of around five seconds, about 35% faster than other devices, Siemens Healthineers CEO Bernd Montag said in a May earnings call.