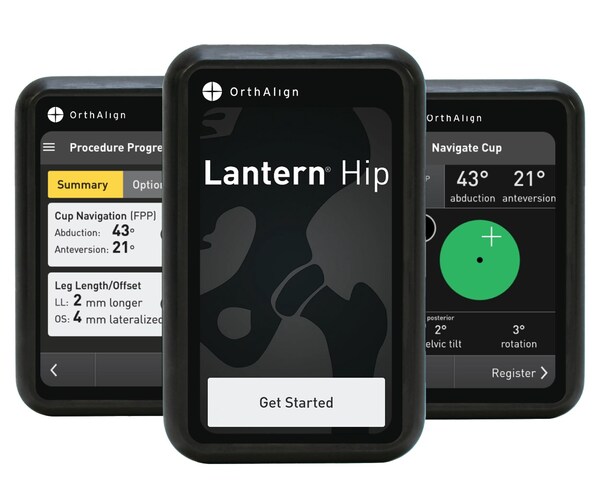
Lantern Hip builds upon the legacy of HipAlign® with enhanced technology and usability. Next-generation accelerometers and gyroscopes offer orthopedic surgeons a streamlined workflow, real-time navigation for cup positioning, and restoration of leg length and offset. The technology enables individualized cup positioning compared across multiple planes, including the functional pelvic plane, anterior pelvic plane, and coronal plane, with live pelvic tracking. Designed for personalized patient care, Lantern Hip eliminates the need for pre-operative imaging or capital equipment and is compatible with most implant systems, making it an ideal choice for the ASC setting.