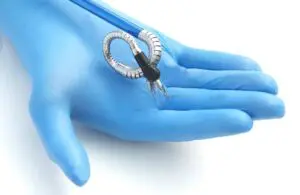
Anovo received clearance for use in single-site, abdominal access across ventral hernia repair. The clearance complements Anovo’s existing approvals for natural orifice laparoscopic-assisted transvaginal benign gynecology procedures.
Momentis designed Anovo as a single-port robotics platform and clearance makes it the first such system cleared for this indication. The company says procedures performed through a single port with multiple flexible instruments could potentially result in less invasive and less traumatic outcomes for the tissue at point of entry.