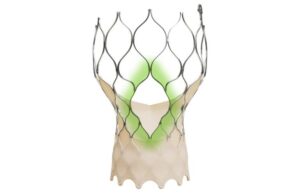
The medtech giant received FDA approval for the same system in March of this year. Both FDA and CE mark approval cover the treatment of symptomatic severe aortic stenosis. It says the latest version of the Evolut valve maintains the performance of the Evolut platform with a design that facilitates coronary access.
Evolut FX+ TAVR offers three larger coronary access windows through a modified diamond-shaped frame design. The access windows are four times larger than previous iterations of the Evolut system. This design provides increased space for catheter maneuverability, facilitating access to coronary arteries in varying anatomies.