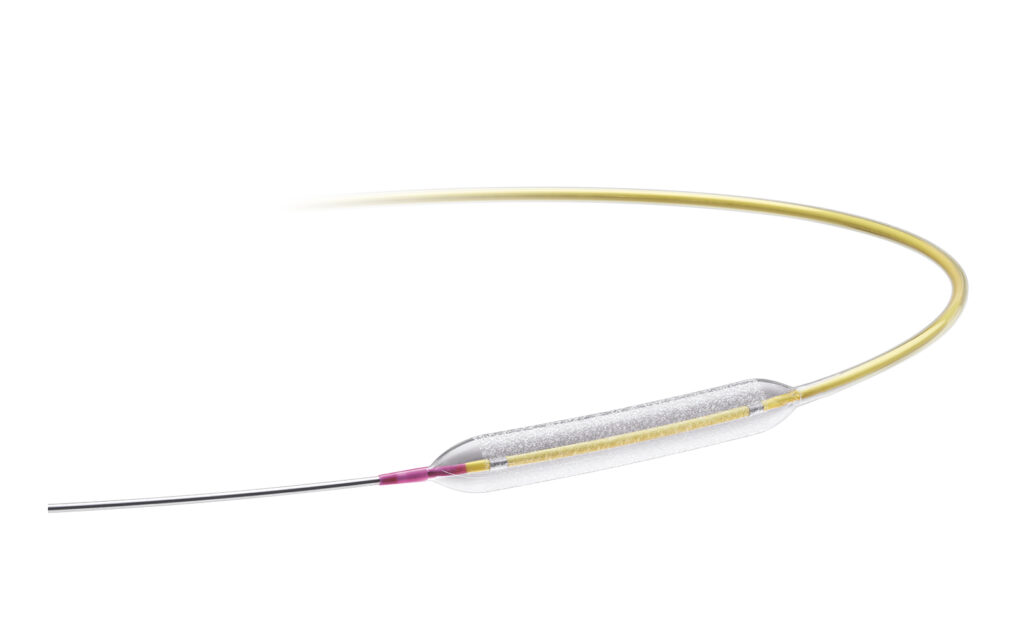
The medtech giant can now begin a pivotal clinical trial for the coronary paclitaxel DCB for in-stent restenosis (ISR) and de novo small vessel disease. It plans to use data from its Prevail Global Clinical Program to support approval efforts in Japan and the U.S. Prevail currently has availability in more than 79 countries, including across Europe, where it launched in 2021.
Medtronic said expects the multi-center, dual-cohort trial to enroll up to 1,205 patients with coronary artery disease. It takes place across approximately 65 global centers in the U.S., Europe and Asia Pacific. The trial includes a randomized controlled evaluation of ISR patients and a single-arm evaluation of de novo small vessel patients. Ultimately, it aims to assess the safety and efficacy of the Prevail DCB.