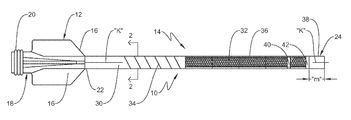
San Jose, California-based Vantis develops the CrossFast system along with the CrossShock intravascular lithotripsy (IVL) system. It designed CrossFast to help physicians perform faster, easier and safer procedures. The guide extension catheter offers additional support and facilitates device delivery in challenging anatomies and complex, high-risk cases.
According to Vantis, limitations of traditional guide extension catheters include insufficient pushability and risk of vessel trauma. CrossFast features patented DuoPro interlocking technology that couples the outer and inner extension catheters. This offers safety and deliverability, enhancing interventional procedures from start to finish.