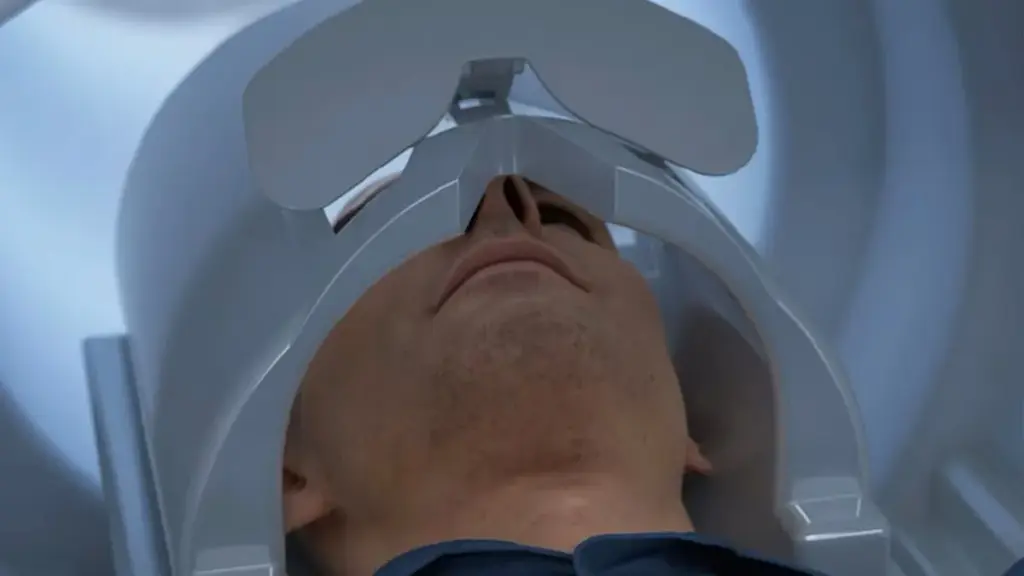
MRI scans of the head can inform treatment of neurological diseases, cancers and psychiatric conditions. Traditionally, whole-body scanners are used to capture images of the head. The procedure requires the patient to lie still in a narrow, enclosed space for 30 to 60 minutes. Head-only scanners, which multiple groups have researched, could make the experience less traumatic while realizing other benefits.
The Food and Drug Administration clearance positions GE to find out if there is a market in the U.S. for such a device. GE has redesigned the coil for neuroimaging, resulting in a system the company says offers enhanced spatial resolution and image clarity to enable the detection of subtle abnormalities.
GE has installed investigational Magnus systems at the Walter Reed National Military Medical Center, University of Iowa, University of Wisconsin – Madison, and Brigham and Women’s Hospital. In research settings, the systems could support the discovery of biomarkers for neurodegenerative disorders and aid other projects.