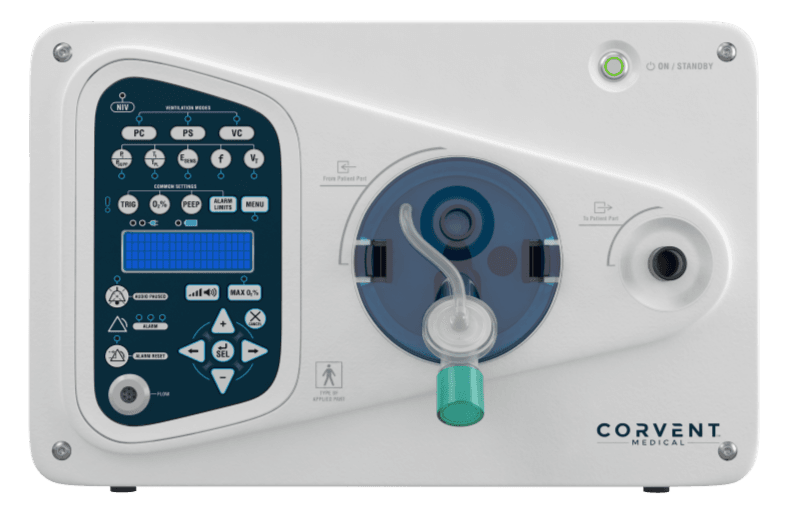
FARGO, N.D., Nov. 20, 2024 /PRNewswire/ — CorVent Medical, a medical device and mechanical ventilator manufacturer, announced that its RESPOND ventilator has received U.S. FDA 510(k) clearance. The RESPOND Ventilator is cost-effective and designed to deliver simple, safe and smart ventilation, expanding access to quality care for healthcare systems, providers and patients.
The RESPOND comes with a 5-year Limited Warranty, an industry first, making the total cost of ownership the purchase price. The system is a lightweight and easy-to-use ventilator that combines proven elements of traditional mechanical ventilation with durable, long-lasting components, eliminating the need for service and maintenance contracts. The RESPOND ventilator is a full-featured ventilator, which uses non-proprietary circuits and accessories along with low-pressure oxygen for long-term affordability.
“Simple and highly reliable, the RESPOND ventilator is a front-line ventilator in a value-focused and resource-constrained world,” said Patrick Troy, MD., chief medical officer, CorVent Medical. “Its robust design maximizes total cost of ownership, delivering significant savings for hospital systems, emergency stockpiles, long-term acute care hospitals (LTACHs) and skilled nursing facilities (SNFs).”