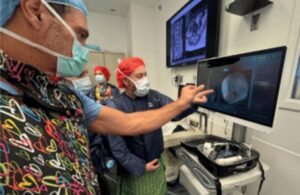
The Xvision Spine System uses augmented reality to give surgeons “X-ray vision” during surgery. It launched new, FDA-cleared features and indications for the system in March. This latest addition enables surgeons to navigate off a preoperative CT scan and fluoroscopic images from a standard 2D C-arm. That eliminates the need for 3D intraoperative imaging.
Augmedics also reported the first surgical case completed using this new registration. Dr. Frank Philips completed this case at Rush University Medical Center in Chicago. Philips previously completed the world’s first augmented reality-navigated minimally invasive spine surgery in 2020.