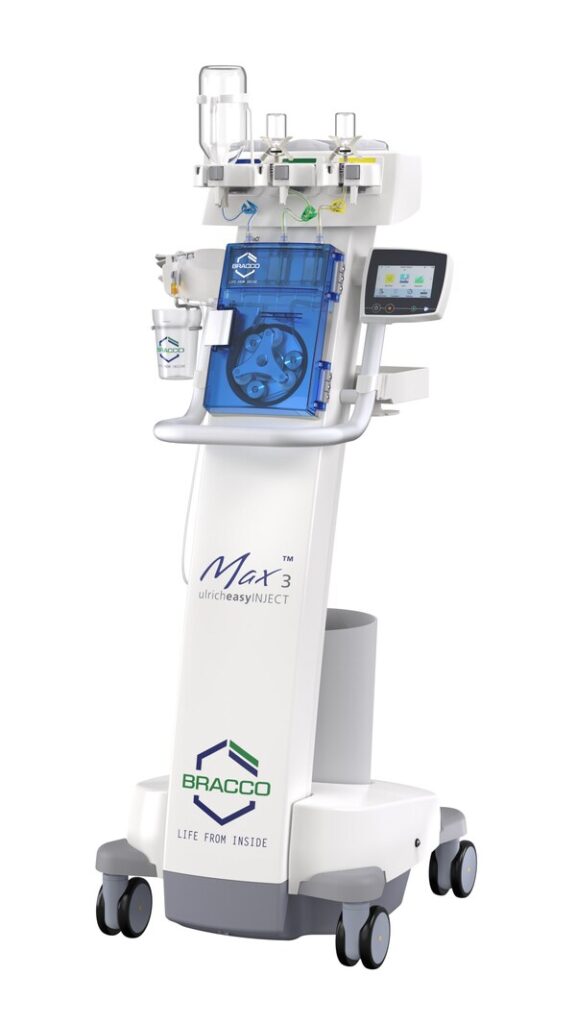
The Max 3™ is a first of its kind in the MRI space. The innovative injector offers intuitive, efficient, and easy-to-manage features such as direct injection from original contrast media vials meaning no need for refilling of syringes, and no power cables so it can be positioned anywhere in the MRI room up to a maximum magnetic field strength of 50mT. The Easy-Click-Cassette flex has a dedicated connector to the patient tubing with SafeConnect, providing contact protection and reliable protection against retrograde contamination which can be used within 24 hours or up to a maximum of 96 bottles of contrast media, or whichever comes first, thus improving workflow for the technologist, allowing more time to focus on the patient during a procedure.
In addition to being easy to use, the Max 3™ injector supports best practices in radiology sustainability. This injector will strengthen green efforts in radiology suites because it will contribute less plastic to a hospital’s disposable process and costs.