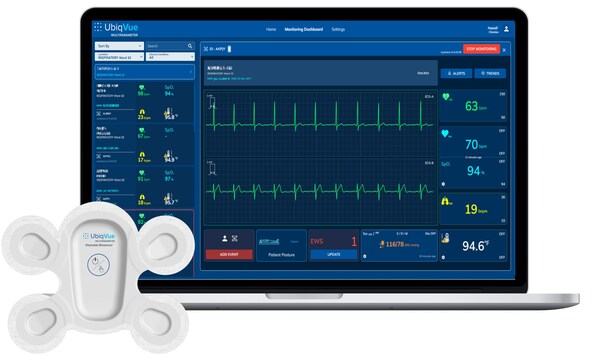
Central to the system is the UbiqVue 2A Biosensor, a single-use, all-in-one wearable device that enables SpO2** to be continuously collected from the chest, alongside other biodata, for generating a total of twelve monitored parameters, including 2-channel ECG, pulse rate, PPG, respiration rate, body temperature, and motion. The encrypted data is securely transmitted, in near real-time, from the Biosensor via a relay app or an access point to a secure cloud-based system, where it is further signal-processed. Healthcare professionals and care providers can access continuous vital signs, via the UbiqVue web portal, and receive alert notifications.
“This Class IIb EU MDR Certification, typically given to devices intended for the continuous surveillance of vital physiological parameters in anesthesia, intensive care or emergency care, confirms our commitment to deliver bedside patient monitor-equivalent functionality through an affordable, single-use Biosensor,” said Surendar Magar, Co-founder and CEO. “By securing the necessary regulatory approvals in various geographies and creating global partnerships with OEMs, service providers, and distributors, we aim to transform healthcare at scale.”