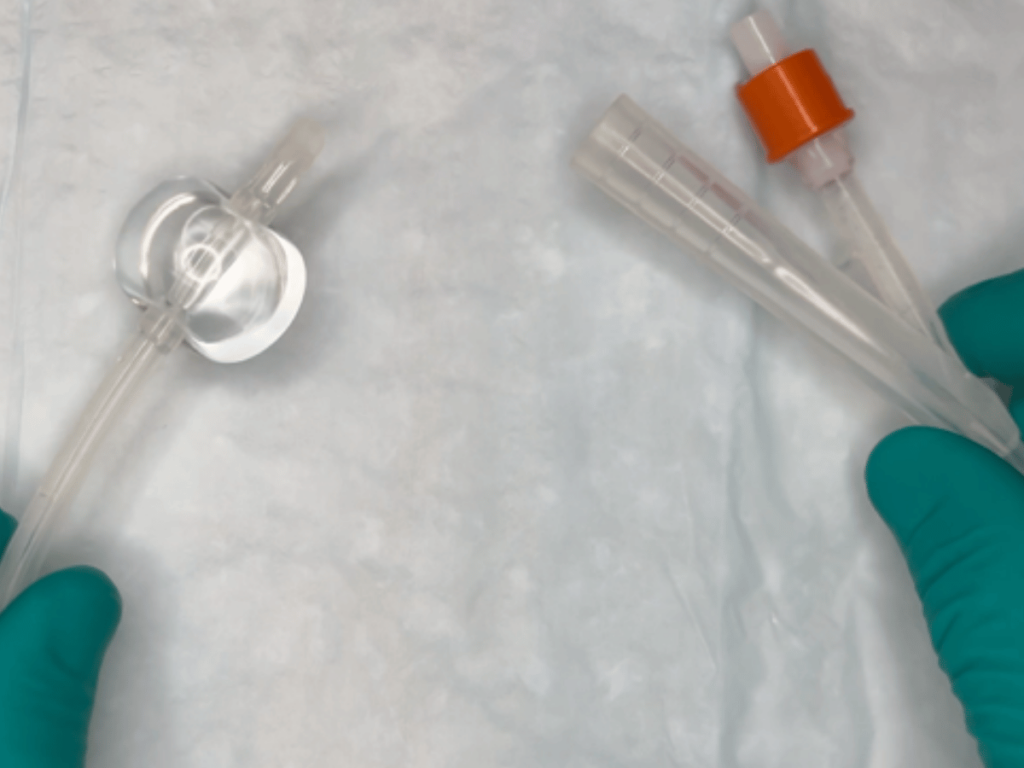
The Miami-based company says it marks the first-ever FDA clearance under a new code for urinary catheters with safety features.
InnoCare designed its Egress safety catheter to eliminate the injuries that occur when traditional indwelling catheters are pulled out while the catheter balloon is still inflated. The company’s proprietary technology enables the catheter balloon to automatically deflate before a pullout occurs. This helps to avoid extensive patient harm.
According to a news release, clinicians can seamlessly integrate Egress into clinical practice without additional supplies or preparation. Dr. Bruce Gardner, a radiologist at Sanford Health, said a pulled-out catheter can typically lead to significant bleeding, extended catheterization time and reparation surgery. Gardner called Egress a “safer alternative,” especially for patients in the ICU and those with delirium or dementia.