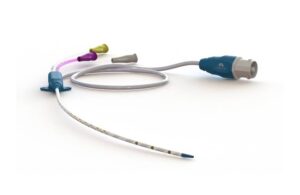
AeroPace uses neurostimulation via an electrode-containing cardiovascular catheter and a software-controlled system. It delivers periodic phrenic nerve stimulation through the venous catheter. The system contracts and strengthens the diaphragm in patients on mechanical ventilation.
Exton, Pennsylvania–based Lungpacer’s system received an FDA indication to improve weaning success. It aims to increase weaning, reduce ventilator days and reduce reintubation for patients aged 18 years or older on mechanical ventilation for 96 hours or longer without weaning.
The company said in a news release that this marks the first FDA nod for a device of this kind.